
Discovering the potential of NMP
The Türker Lab explores how neuronal membrane proteasomes regulate neuronal signaling and contribute to neurodegenerative diseases like Huntington’s, aiming to uncover novel mechanisms and therapeutic targets.

The Türker Lab explores how neuronal membrane proteasomes regulate neuronal signaling and contribute to neurodegenerative diseases like Huntington’s, aiming to uncover novel mechanisms and therapeutic targets.
“Proteasomes are more complex than appreciated”
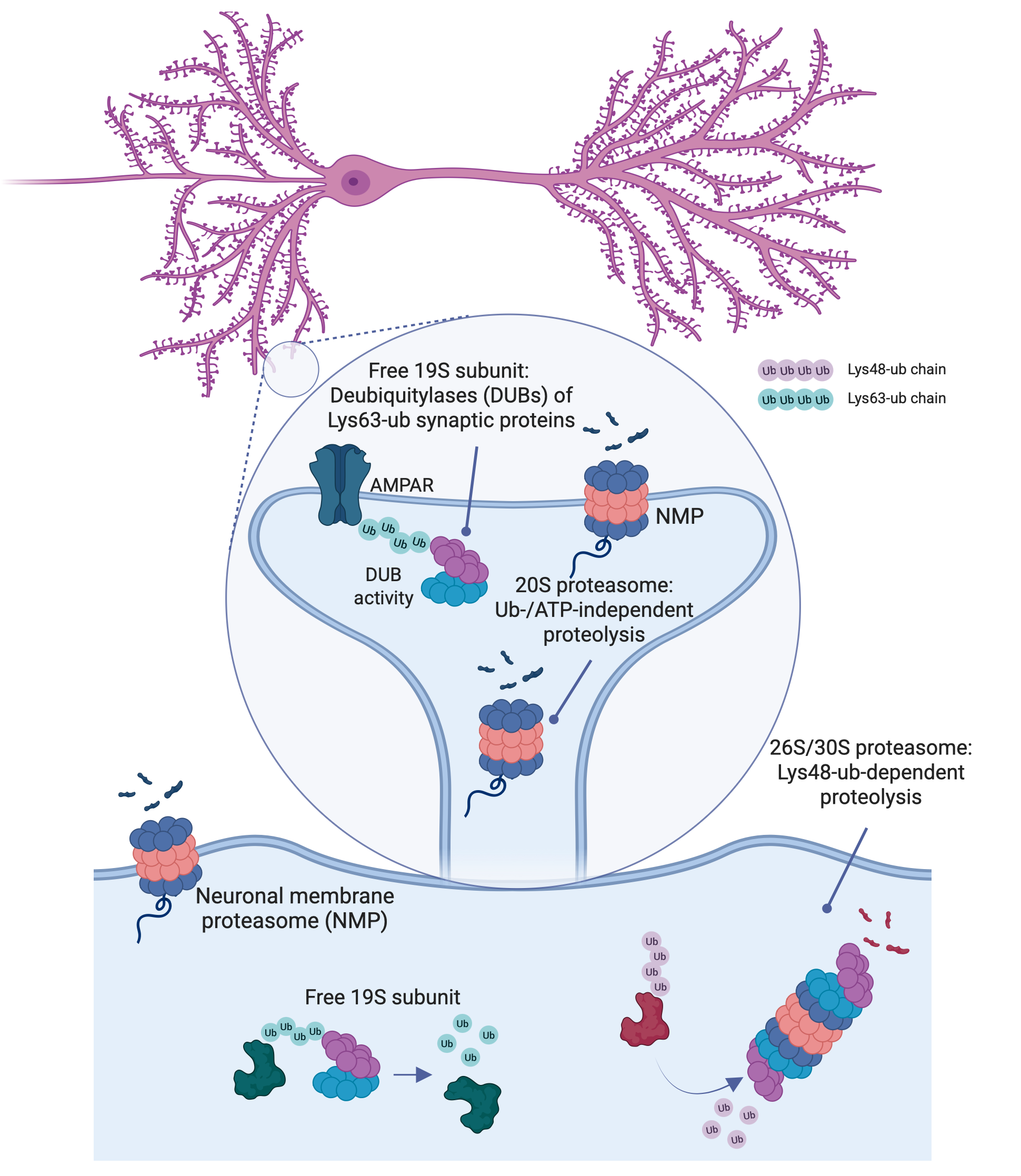
Proteasomes are the main machinery for protein degradation in all eukaryotic cells, breaking down proteins into short peptide fragments typically 3–30 amino acids in length. Proteasomes are highly dynamic and multifunctional macromolecular complexes, involved not only in maintaining proteostasis but also in regulating signaling, apoptosis, immunity, and synaptic plasticity. Their subunit composition and subcellular localization endow them with specialized roles across different contexts.
In neurons, a distinct pool of proteasomes localizes to the plasma membrane, where they degrade intracellular proteins and release bioactive peptides directly into the extracellular space. These neuronal membrane proteasomes (NMPs) have been shown to regulate neuronal signaling, experience-dependent plasticity, and sensory responses such as pain. Recent findings also point to a dysregulation of this membrane-localized proteasome complex in neurodegenerative diseases.
Our goal is to investigate the signaling functions of NMPs and to understand how their disruption contributes to Huntington’s disease progression. Our lab is among the few currently focused on uncovering the mechanistic and functional relevance of NMP biology. Given the well-established connection between proteasome dysfunction and disease, we aim to define the role of this unique, neuron-specific membrane proteasome in the context of neurodegeneration.

“For most of history, Anonymous was a woman.”
-Virginia Woolf